Overview
This US-based research company, founded 15 years ago, specializes in providing competitive intelligence to pharmaceutical and medical device companies across the globe. The research company’s services range from intelligence gathering to providing a market landscape that includes tracking of tactical and strategic activities of competitors.
Key Challenges
The research company aimed to track the market and competition for a US-based pharmaceutical client. The client wanted to monitor the competition to improve its existing portfolio. This involved tracking regulatory databases, news sources, and competitor’s earning calls, and more. The client also required instant alerts on any important news or market development along with the implications of that news or market development on their business.
Before Contify, the research company conducted manual tracking of the client’s competitors’ company websites, news sources, and regulatory databases. This manual process was inefficient, labor-intensive, and time-consuming. As a result, the research company was looking for a competitive intelligence tool that leverages Artificial Intelligence to automatically track strategic updates on key competitors of the client. The research company’s requirement also includes:
- Tracking live coverage of the client’s competitors earning calls and its summary
- Aggregating strategic updates from regulatory databases in the US and Europe, specifically country-wise regulatory databases in Europe
- Automating the monitoring and tracking of news updates on the strategic developments of the client’s competitors
- Creating a customized weekly intelligence report with actionable business insights
Contify’s Solution
The research company conducted adequate due diligence across different market intelligence software providers. Contify a leading market and competitive intelligence solution was selected to meet their requirements. Contify team analyzed the requirements of the research company in detail and deployed a customized AI-enabled market intelligence solution that can deliver real-time updates.
Customized Categorization of Market Signals
Contify team coordinated with the research company and the client to prepare a detailed list of the pipeline drugs, and competitors. The team also created company profiles for all the competitors identified in due consultation with the client and the research company. The profiles focused on highlighting competitors’ expertise in the market, details regarding their financial performance, manufacturing abilities, recent news related to new offerings and product development, and insights from primary research.
Customizing Platform Configuration:
Contify tailored its contextual noise-filtering and machine learning algorithms to share automated updates and news feeds with the research company. The newsfeeds presented the strategic updates in a structured manner with tags customized for the client’s business objectives, competitors, and products. Different news channels, websites of competitors were also added to the competitive intelligence platform to enable sourcing of information from the relevant sources. Custom configuration was done to filter out the irrelevant content. AI models were re-trained to enable the exclusion of news related to products that do not belong to the product portfolio of the client.
Monitoring Regulatory Sources:
Most of the client’s competitors publish press announcements about their product approval. However, these press releases do not reveal enough information about the specific regulator and the country that has approved the product. This information was important for the client. To meet this business objective, the Contify platform was configured to track each European country’s regulatory website. As part of Contify’s managed services offerings, Contify’s team of analysts performed a process-driven search on WHO and other websites to find relevant sections on the regulator websites. Contify analysts also used translations of the keywords like regulatory and health in the native language of the country as an input to extract relevant insights. After that, a list of these country-wise databases was tracked daily by Contify’s automated intelligence platform to generate actionable insights.
Tracking Live Coverage of Pharmaceutical Events to Gather Insights:
Under Contify’s managed services live quarterly calls and industry conferences were also covered in which the client’s competitors participated. Contify analysts also shared a summary of the calls along with a precise analysis and opinion, with the research company, enabling efficient monitoring of its client’s competitors.
Creating a Dedicated Newsfeed with Analysis:
Contify aggregated all the strategic news events in one report and shared the daily/weekly market intelligence reports with the research company. The newsfeed customized news updates according to the research company and the client’s requirements. Further, Contify’s managed services team identified the news relevant to the client’s business and do a deep analysis to deliver granular and actionable insights.
Sample updates:
Japan’s PMDA completes GMP inspection of Lupin’s Goa facility (Unit- I & II)
Background and detail
Pharma major Lupin announced the completion of the Good Manufacturing Practices (GMP) inspection of its Goa facility (Unit – I & II), by the Pharmaceutical and Medical Devices Agency (PMDA), Japan. The inspection was conducted between September 24, 2019, and September 27, 2019.
Lupin in May 2019 already had faced a backlash when the USFDA informed that the manufacturing facility will be subject to administrative action and that the USFDA may withhold approvals for newer drugs and pending applications.
Contify opinion
On August 22, 2019, Lupin through its Japanese subsidiary, Kyowa (Kyowa Pharmaceutical Industry Co., Ltd.) entered into a definitive agreement for the sale of its Japanese Injectables business and related assets in Japan to neo ALA Co. Ltd, a wholly-owned subsidiary of Neopharma group, the UAE’s largest pharmaceutical manufacturer headquartered in Abu Dhabi.
The Goa facility is known to manufacture oral and injectable drug products for the US market. With the PMDA approval, the company can tap the opportunities in the Japanese market but since Lupin divested its injectable business in Japan, it is more likely that this approval will help Lupin to expand its presence in Japan with the oral finished formulations.
Impact
Generating Actionable Insights, faster
The AI-based market intelligence platform helped the competitive intelligence research company and its client in reducing the time spent on manual tracking of competitors. The research company analysts could now invest time in other important areas of intelligence activities without worrying about the quality of the secondary monitoring.
Enabling Swift Decision Making in Sync with Market Drivers
Contify’s market and competitive intelligence solution provided the research company with relevant updates of earnings call coverage and weekly intelligence reports. Besides, the Contify team also included a brief and precise analysis of the strategic events about the client’s market landscape. It helped the research company to create a comprehensive solution for the client enabling them to strategize informed decisions based on actionable insights.
“Contify’s easy to use platform enabled our pharma client to anticipate market changes and stay on top of the rapidly changing competitive landscape with detailed insight and intelligence. We’re very happy and positive about our partnership with Contify and look forward to opportunities to expand it even further” – Co-founder, Competitive Intelligence Research Company
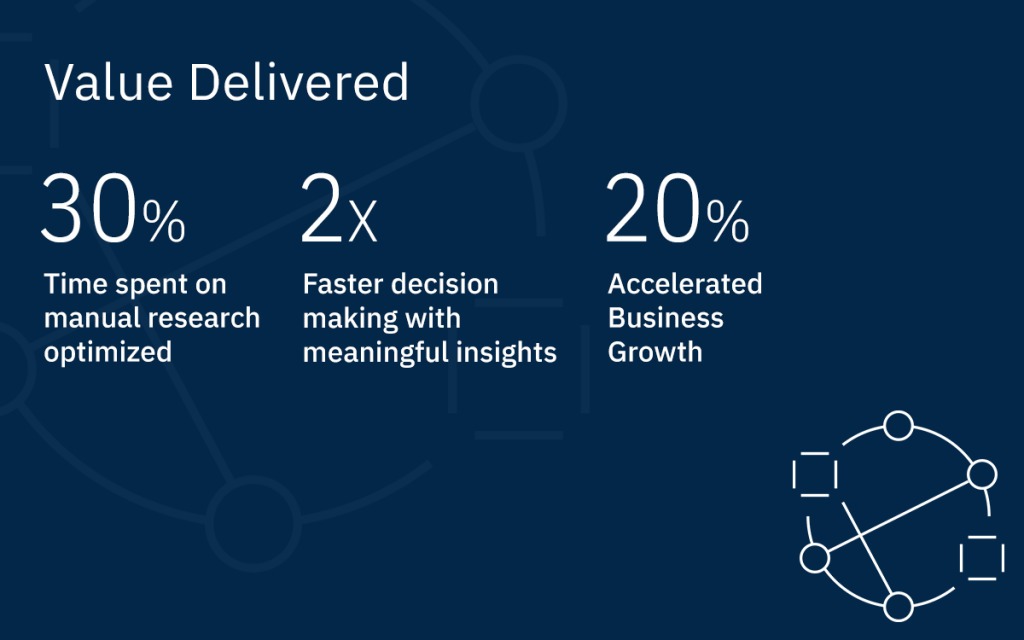
By the Numbers
-
30%
Time-saved in manual research
-
2X
Faster decision making
-
20%
Accelerated business growth






